
受全球疫情的影响,各国的防疫物资出口政策不断在改变,这也让各外贸出口企业疲于应付。美国FDA近日颁布了在新冠肺炎疫情期间口罩的紧急授权(EUA),并发布了相关指南,今天和大家简要说说这份指南的内容。
一、绿色通道适用产品代码
首先,FDA说明了该政策适用的产品代码范围:
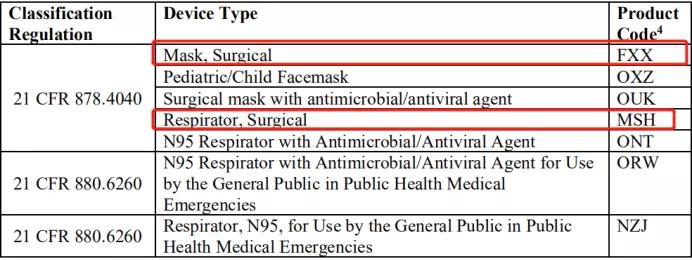
如图所示,可申请的类目包含:外科口罩、儿童口罩、外科防毒面具、N95口罩、呼吸机等防疫物资。
接着FDA说明以下产品代码不在该政策适用范围,有且不限于以下这些:
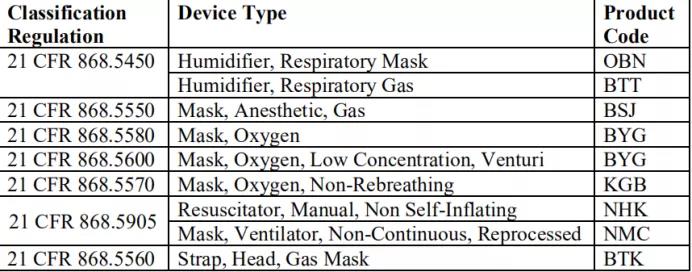
二、医疗器械or非医疗器械
FDA在该指南中解释了什么属于医疗器械,什么不属于医疗器械:
Face masks and respirators are devices when they are intended for a medical purpose, such as prevention of infectious disease transmission (including uses related to COVID-19).
Face masks andrespirators are not devices when they are intended for a non-medical purpose, such as for use in construction.
可以简单理解成有医疗用途的口罩就属于医疗器械,没有医疗用途的口罩就不属于医疗器械。
三、如何申请EUA?未在美国境内的上市的医疗用途的外科口罩、N95口罩,不管是美国境内还是境外制造商,都可以申请紧急授权。需要递交以下资料,并发送到FDA 这个邮箱:CDRH-COVID19-SurgicalMasks@fda.hhs.gov,由FDA审核是否可以授予紧急授权,让该产品在新冠疫情期间在美国境内销售。
资料如下:
1、General information such as your contact information, name and place of business,email address, and contact information for a U.S. agent (if any) in addition to generalinformation about the device such as the proprietary or brand name, model number,and marketing authorization in your country (or region).
2、copy of the product labeling.
3、Whether the device currently has marketing authorization in another regulatory jurisdiction (including certification number, if available).
4、Whether the device is manufactured in compliance with 21 CFR Part 820 or ISO13485: Medical Devices – Quality Management Systems – Requirements forRegulatory Purposes or an equivalent quality system and the manufacturer or importer has documentation of such.
5、Description of testing conducted on the device, including any standards met, such as
6、liquid barrier protection, flammability, biocompatibility, and filtration performance, asappropriate. For surgical N95 respirators, FDA recommends including fluid resistance testing (liquid barrier performance).
以上递交资料适用于本来就已经生产医疗器械、但口罩尚未在美国境内上市的制造商。有两点是需要注意的:
1、FDA只是减免了510(k)技术文档要求,但关于产品测试,生产车间质量体系的要求是从未有放弃的;
2、在新冠肺炎疫情爆发期间内,FDA不拘泥于任何区域的产品标准,只要你是做相关产品测试,欧盟的、中国的,都可以将测试报告递交,由FDA决定是否可以授予紧急授权。
此外,FDA也欢迎非医疗器械企业生产销售医疗器械,可以粗暴理解成就是连生产车间QSR820都未外审过的制造商,至于要怎么操作,发邮件问FDA。
四、额外要求
FDA对于紧急授权下的口罩产品,有如下要求:
Appropriate conditions designed to ensure that health care professionals administering the device are informed—that FDA has authorized the emergency use of the device;
of the significant known and potential benefits and risks of the emergency use of the device, and of the extent to which such benefit and risks are unknown;
of the alternatives to the device that are available, and of their benefits and risks.
Appropriate conditions designed to ensure that individuals to whom the device is administered are informed—
that FDA has authorized the emergency
of the significant known and potential benefits and risks of the emergency use of the device, and of the extent to which such benefit and risks are unknown;
of the option to accept or refuse administration of the device, of the consequence,if any, of refusing administration of the device, and of the alternatives to the device that are available and of their benefits and risks.
获得EUA授权的口罩,要在包装标识上明确这是FDA紧急授权的产品。
官方操作指导链接如下:https://www.fda.gov/regulatory-information/search-fda-guidance-documents/enforcement-policy-face-masks-and-respirators-during-coronavirus-disease-covid-19-public-health
想要了解更多关于最新口罩认证资讯内容,请登录PROMISE检测官网:www.pnms-test.com或拨打咨询热线:0755-23319501。
PROMISE检测您值得信赖的检测品牌!

